Zirconium Mullite Products
Sintered zirconium mullite products are advanced refractory materials made by introducing ZrO2 into mullite matrix through reaction sintering process using industrial alumina and zirconium concentrate as raw materials. The reaction equation is as follows:
3Al2O3+2ZrSiO4→3Al2O3·2SiO2+2ZrO2
Mullite ceramics have many excellent properties, such as high temperature resistance, thermal shock resistance, high temperature creep resistance and good chemical stability. By introducing ZrO2 into mullite ceramics, the mechanical properties of mullite materials at high temperature can be greatly improved by means of phase transition toughening of ZrO2. ZrO2 is promote the sintering of mullite material, the addition of ZrO2, due to its low melting material produced or vacancy formation can accelerate the densification of ZTM materials sintering process, when the mole fraction of 30% of ZrO2, at 1530 ℃ to burn into the body of the relative volume density was 98%, and the strength of 378 MPa toughness reaches 4.3 MPa m1/2. The different composition of ZTM material, its strength mechanism is different. When the volume fraction of ZrO2 was 15%-30%, ZTM was mainly stress-induced phase transition toughening. When the volume fraction of ZrO2 is more than 30%, the toughness of ZTM material is mainly caused by microcrack. The particle size of ZrO2 powder has great influence on the mechanical properties of ZTM material. ZrO2 has a large particle size. Most of the ZrO2 in the matrix of ZTN material is in the form of m-zro2. When the particle size of ZrO2 is small, the relative content of t-zro2 in ZTM material is high, and the improvement of mechanical properties of the material is mainly due to the toughening of t-zro2 by phase transition.
There are two production processes to produce sintered zirconium mullite products. The other is a partial phase transition that maintains a nonequilibrium phase combination. The two production processes have their own characteristics and can produce products with excellent performance.
In industrial production, high bauxite is often used instead of industrial alumina to reduce costs to produce mullite products containing zirconium.
Zirconium mullite products made of industrial alumina and zirconium
Zirconium mullite products were prepared by reaction sintering using alumina and zirconium as raw materials. Usually, the first in the 1450 ℃ heat when burnt, make its densification, then heat up to 1600 ℃, reaction, ZrSiO4 in more than 1535 ℃ is decomposed into ZrO2 and SiO2, the SiO2 and Al2O3 combining produce mullite, due to the decomposition of ZrSiO4 for SiO2 and ZrO2, part of the liquid phase, and the decomposition of ZrSiO4 can make further fragmentation particles, increase the active surface and promote the sintering.
The quantitative analysis results of X-ray diffraction showed that, when the temperature was higher than 1380℃, a certain amount of amorphous phase existed in the sample. There are two main ways to produce amorphous phase: first, due to the presence of impurities, the system produces liquid phase at a lower temperature; Second, the chemical reaction of ZrSiO4 and Al2O3 produces the amorphous phase. There are two views on the properties of the amorphous phase. Some people think that the amorphous phase has the chemical composition of mullite, which is amorphous mullite. It is thought to be amorphous SiO2 or an amorphous phase rich in SiO2. In the temperature range of 1400~1440℃, the content of amorphous phase is relatively high. However, when the temperature increases, the content of amorphous phase decreases. This is because most amorphous phases are intermediates of chemical reactions that, when the temperature rises, further react or crystallize to form phase products.
The clinker of zirconium corundum mullite was synthesized from industrial alumina (99.5% Al2O3) and zirconium (ZrO2 65.9%). According to the theoretical calculation, when the zircon content is 54.7%, all the introduced Al2O3 and SiO2 just completely react to form mullite (A2S2). In the range of zircon content less than 54.7%, with the increase of zircon content, the microstructure of sintered samples is a network structure composed of columnar mullite. The flexion strength of the sample at high temperature (1400℃) first increased with the increase of ZrO2, and then decreased when the content of ZrO2 was 23.7%. The addition of zircon contributes to the improvement of thermal shock resistance.
Zirconium mullite products made of high alumina and zirconium
With high quality bauxite clinker and zirconium as raw materials, made of zirconium mullite products, such as anti - peeling high aluminum bricks, wear resistance and thermal shock resistance, the addition of zirconium also improve the anti - alkali erosion of high aluminum materials. Such products have been used in cement rotary kiln and glass melting kiln.
Ⅰ etc high and Ⅱ shanxi bauxite base makings, add 10% ~ 15% of guangdong zircon (ZrO2 64%), the zircon addition type of diaspore, kaolinite (DK) high sintered bauxite phase composition, microstructure and high temperature mechanical properties. The results showed that zirconium reacted with corundum at 1400℃ to produce monoclinal ZrO2 and cubic mullite, and there were microcracks around the monoclinal ZrO2 crystal. The crystal development of tertiary mullite and the solid solution of TiO2 and Fe2O3 were lower than that of secondary mullite. Ⅰ sintered bauxite, such as the introduction of zircon, because part of corundum mullite and monoclinic ZrO2 replaced by mullite network structure has been improved to a certain. Ⅱ after sintered bauxite in zircon, mullite decreases, monoclinic ZrO2, glass phase increases. The changes of rigidity modulus, shear strength, flexural strength and compressive creep were measured at different temperatures. The results showed that the rigidity modulus and strength decreased and the total deformation increased with the addition of zircon before 1200℃. At 1300~1400℃, when the zircon content exceeds 30%, the rigidity modulus and strength slightly recover. This is closely related to the change of phase composition and microstructure. In terms of crystallization effect, cracks around monoclinic ZrO2 play an important role in weakening. In the glass effect, the glass phase viscosity at 1300~1400℃ was significantly increased due to the increase of zircon content.
The dense AZS clinker was prepared by sintering with special grade bauxite (Al2O3 89.3%) and zircon (ZrO2 65.8%). The ratio of ingredients: special grade bauxite 54%, zircon sand 46%, clinker porosity of 10%~18%, volume density of 3.08~3.21g/cm3. In sample preparation, the proportion of mud particles: 2~0.5mm thick particles 50%, less than 0.5mm medium particles 10%, less than 0.074mm fine powder 40%. The waste pulp liquid was used as the binder, the molding pressure was 147MPa, the firing temperature was 1600℃, and the heat preservation was 4h. X-ray diffraction analysis of the sample after firing showed that mullite was the main mineral phase, monoclinal ZrO2 was the second, and there was a small amount of tetragonal ZrO2. It can be seen from the SEM photos of the sample fracture that ZrO2 is evenly distributed in the matrix, and the matrix is well combined with particles. The porosity of the samples is 17%-21%, the volume density is 2.89-3.02g /cm3, the compressive strength at room temperature is 60-80mpa, the bending strength at room temperature (1400℃,0.5h) is 8-12mpa, the softening temperature under load starts at 1580-1590 ℃, the resistance to rapid cooling and heating (water cooling at 1100℃) is greater than 13 times, and the residual bending strength after air cooling at 1100℃ is 27-35mpa.
In the study of the effect of rare earth oxides (REO) on AZS material sintering and microstructure, it was concluded that :(1) the addition of rare earth oxides La2O3 or CeO2 alone can promote AZS material sintering. Under the condition of calcination for 4h at 1550℃, with the increase of the amount of REO, the degree of promoting sintering increased. Under the condition of calcination at 1600℃ for 4h, adding 0.5%REO can significantly promote sintering. Increase the content of REO, the effect is not good. (2) the effect of adding appropriate amount of REO on the structure of azs-40 material is as follows: the structure of azs-40 material is dense, and the grain distribution of ZrO2 tends to be uniform. However, if too much (more than 1%) is added, the grain size of ZrO2 will grow and the glass phase will increase. (3) influence of comprehensive REO on sintering performance and microstructure of al2o3-zro2-sio2 material; the appropriate amount of REO is 0.5%~1.0%.
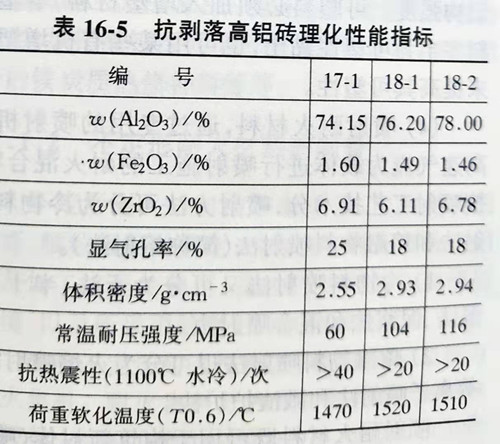
The addition of ZrO2 has an obvious effect on improving the thermal stability of high aluminum bricks. The research shows that ZrO2 is introduced in the form of ZrSiO4 with high bauxite clinker as the basic raw material, reasonably graded and a small amount of admixture is added, which is mixed, formed and fired. Zircon was prepared by adding 5%~15% zircon and firing temperature was 1500℃. Its performance is shown in table 16-5.
The microstructure characteristics of the anti-spalling high aluminum brick are as follows: there are obvious microcracks around the ZrO2 aggregate, and the aggregate has obvious "gap peeling" structure and morphology with the surrounding corundum and mullite. The well-developed ZrO2 aggregate was prismatic and distributed in the spatial cambium. Further observation showed that there were very small slits between ZrO2, with the size of only 1-5 cm, forming a channel structure. This kind of structure is in favor of stress transfer and dispersion obviously. Mullite is well developed and fibrous, interlaced in space with ZrO2 and corundum, forming a composite reinforcement structure. After decomposition at high temperature, ZrSiO4 reacts with the surrounding Al2O3 to form mullite. The presence of ZrO2 crystals prevents the growth of fibrous mullite crystals and corundum crystals, and thus presents a chaotic state around ZrO2.
The thermal shock resistance mechanism of ZrO2 is as follows: the monoclinic-tetradectic phase transition of ZrO2 occurs at high temperature, with a volume change of 4%~5%, and microcracks occur at and around the tip of ZrO2. These micro-cracks can make the stress at the front of the main crack change and absorb the fracture energy during the process of loading. The difference of linear expansion coefficients of corundum mullite and other minerals leads to the mismatch of thermal expansion and cold shrinkage and the formation of microcracks. The thermal expansion mismatch and elastic modulus mismatch of ZrO2 particles and matrix can redistribute the load and improve the bearing capacity of products. ZrO2 particles dispersed in the matrix play a role of nailing to some extent. The slip of dislocation is inhibited and the fracture resistance is improved.
Electrofused particles combined with zirconium mullite products
The bauxite and zirconium are pressed into blank, calcined and then broken to a certain particle size, and then made into zirconium mullite frit by electric melting, and then made into zirconium mullite products by sintering refractory production technology. Such products have been used in heating furnaces, petroleum cracking furnaces, glass kilns, etc.
Using fused AZS waste brick as the main raw material, match with AZS complex binder and zircon sand, developed at the AZS sintered brick with high refractoriness under load (1690 ℃) and (264 mpa), crushing strength, low porosity (4%), 1500 ℃ with liquid sodium calcium silicon glass erosion resistance between fused AZS and fused mullite brick, on some kiln used alternative fused AZS brick.

