Phosphate Castable
The castable refractories prepared with phosphate or polyphosphate as binders and refractory aggregates and admixtures (coagulants) are called phosphate combined castables. The phosphate binders used in the preparation of castables can be divided into two categories: one is acidic phosphate binders, such as aluminum dihydrogen phosphate Al (H2PO4) 3, and phosphate H3PO4, which are mainly used as binders for neutral or acidic refractories. Such as silicon, clay, high aluminum, jade, zirconium mullite, zirconium jade castable binder; The other is polyphosphate binder, such as sodium tripolyphosphate NO5P3O10, sodium hexametaphosphate (NaPO3) 6, mainly used as the basic refractory binder, such as magnesium, magnesium aluminum castable binder binder.
(1) acid phosphate binding castable, acid phosphate in the room temperature with acid and neutral refractory reaction or reaction rate is very slow, so its coagulation and hardening effect is achieved by adding the chemical reaction between the coagulant and acid phosphate. The coagulants used are MgO, CaO·Al2O3, CaO·2Al2O3, NH4F, ZnO, NaCl, talc, etc. However, in amorphous refractory materials, MgO and CaO·Al2O3 are most commonly used. When MgO is used as coagulant, the following hardening reaction will occur with aluminum dihydrogen phosphate.
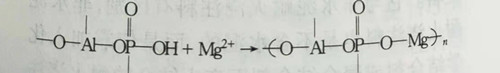
Aluminum-magnesium complex phosphate is formed and hardened by reaction. However, MgO is an alkaline oxide, which has a strong reaction with acidic phosphate, resulting in instant coagulation. The finer the particle, the more intense the reaction. Therefore, in order to control the coagulation and hardening time, the fineness and addition amount of MgO must be strictly controlled.
When CaO·Al2O3 and CaO·2Al2O3 are used as coagulants, calcium aluminate phase is firstly hydrolyzed, and the dissociated CaO reacts with acidic aluminum phosphate to form a composite phosphate of aluminum and calcium, resulting in hardening. The rate of hardening is also controlled by the fineness of calcium aluminate phase and the amount added.
Acid combined aluminum phosphate castable is similar to ordinary castable in grain size distribution and binder liquid aluminum dihydrogen phosphate and phosphoric acid, is commonly used in solution proportion control in 1.4 ~ 1.45, the adding amount was 13% ~ 15%, but due to the liquid aluminum dihydrogen phosphate and phosphoric acid at room temperature and refractory aggregate of metal iron (broken shattered into) H2 gas, and bloating in the lining of the body after pouring shape, and form a porous loose body. Therefore, it is necessary to add anti-swelling inhibitor (concealed agent), or add liquid aluminum dihydrogen phosphate or phosphoric acid twice in the mixing of pouring materials, the first time about 6%~7% phosphate solution into the mixing of good pouring materials mixture, after mixing evenly, the semi-wet mixture trapped for 24h, so that metal iron and acid reaction to produce iron phosphate. Before adding phosphate solution for the second time, first add about 2%~3% of the coagulant, mix the mixture well, and then add the remaining 6%~7% of phosphate solution, and then stir evenly, then pour molding, so as to avoid the occurrence of swelling. Table 17-7 shows the physical properties of aluminum silicate castable bonded by aluminum dihydrogen phosphate.
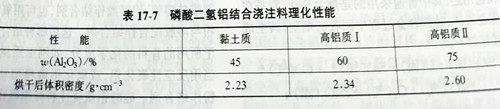
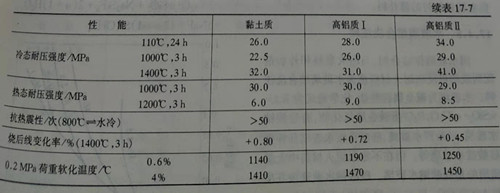
Compared with calcium aluminate cement combined castable, acid phosphate combined castable has better cold compressive strength and thermal shock resistance, so it is generally used for industrial furnace lining with frequent temperature fluctuations and medium temperature wear-resistant lining, and also for hot repair.
(2) polyphosphate is combined with the castable. The polyphosphate, which is dissolved in water at room temperature, will react with MgO or CaO in the alkaline refractory powder and generate composite phosphate to harden the material. However, the reaction speed is relatively slow, so it is suitable to be used as the binder of alkaline refractory castable without adding coagulant. The hardening process is as follows: firstly, polyphosphate is dissolved in water and gradually hydrolyzed to produce dihydrogen phosphate and monohydrogen phosphate. For example, the hydrolysis reaction of sodium tripolyphosphate is as follows:
Na5P3O10+2H2O→2Na2HPO4+NaH2PO4
Then, dihydrogen phosphate and monohydrogen phosphate react with MgO or CaO to form complex phosphates and harden.
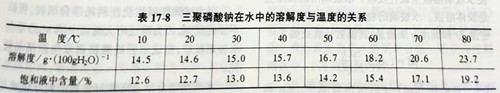
However, the solubility of sodium tripolyphosphate in water is related to temperature, and the solubility increases with the increase of temperature, as shown in table 17-8. Therefore, in order to increase the addition amount, it is necessary to appropriately increase the water temperature, which can accelerate the reaction between polyphosphate solution and MgO in alkaline refractory, thus accelerating the hardening rate.
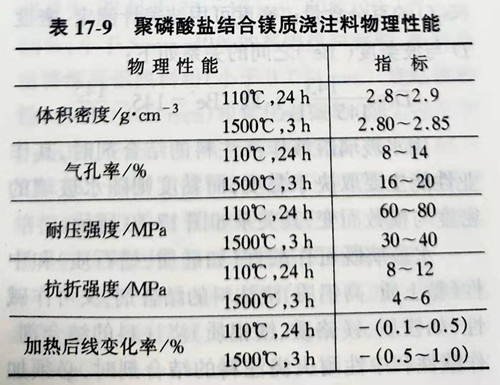
But powdery sodium hexametaphosphate is easy to dissolve in water at normal temperature, and can be mixed with water in any proportion. The viscosity of its aqueous solution decreases with the increase of temperature. The hydrolysates also tend to react with MgO or CaO to form complex phosphates and harden. However, the polymerization degree n of sodium hexametaphosphate has an effect on the bond strength of the castable. In addition, in order to improve the high-temperature strength of the basic castable, a small amount of CaO is added to the ingredients. Na2O·2CaO·P2O5 can be generated at high temperature and then added, which helps to improve the high-temperature strength of the basic castable. Table 17-9 shows the physical properties of magnesium castable with polyphosphate binding.
Polyphosphate combined alkaline castable can be used as lining for high-temperature melting furnace, high-temperature liquid metal container and flow tank, etc., as well as repair material for lining of electric arc furnace.

