Magnesia Charcoal Brick
Magnesium carbon (MgO-C) brick is a kind of unburned carbon composite refractory, which is composed of high melting point alkaline oxide magnesium oxide (melting point 2800 ℃) and high melting point carbon material which is difficult to be infiltrated by slag. All kinds of non-oxide additives are added and carbon binder is used to combine magnesia carbon brick with high melting point basic oxide magnesium oxide (melting point 2800 ℃) and high melting point carbon material which is difficult to be infiltrated by slag.
Magnesia-carbon brick is mainly used in converter, AC electric arc furnace, DC electric arc furnace lining, ladle slag line and so on.
Term
Carbon, charcoal and coal
"charcoal" is the name of the element, the general name of all atoms with a nuclear charge of 6, and the element symbol C. It is often used in writing the simple substance of a carbon element and the name of a compound containing a carbon element. Such as carbon, carbon-60, carbon monoxide, carbon dioxide, carbonic acid, calcium carbonate, hydrocarbons, etc.
"carbon" (carbon) is the name of the element, the general name of all atoms with a nuclear charge of 6, and the element symbol C. It is often used in writing the simple substance of a carbon element and the name of a compound containing a carbon element. Such as carbon, carbon-60, carbon monoxide, carbon dioxide, carbonic acid, calcium carbonate, hydrocarbons, etc.
Carbon is the most important component of charcoal. In addition to carbon, there are some impurities in the carbon, that is, the ash contained in the raw material. Pure carbon should be completely carbon dioxide after burning, with no ash. "Coal" (coal) is a complex mixture of organic and inorganic elements dominated by carbon. In the case of isolated air heating, coal can be decomposed, and the solid substance in the product is coke.
It can be seen that charcoal, activated carbon and coke are simple substances formed by carbon elements, in addition, diamond and graphite are also simple substances formed by carbon elements.
There are various forms of carbon, such as diamond, graphite, fullerene and carbene in terms of its structure; amorphous structure, such as amorphous carbon film, diamond-like film; nanostructured carbon, such as carbon nanotube and carbon nanocluster; and disordered graphite, as shown in table 13-1.
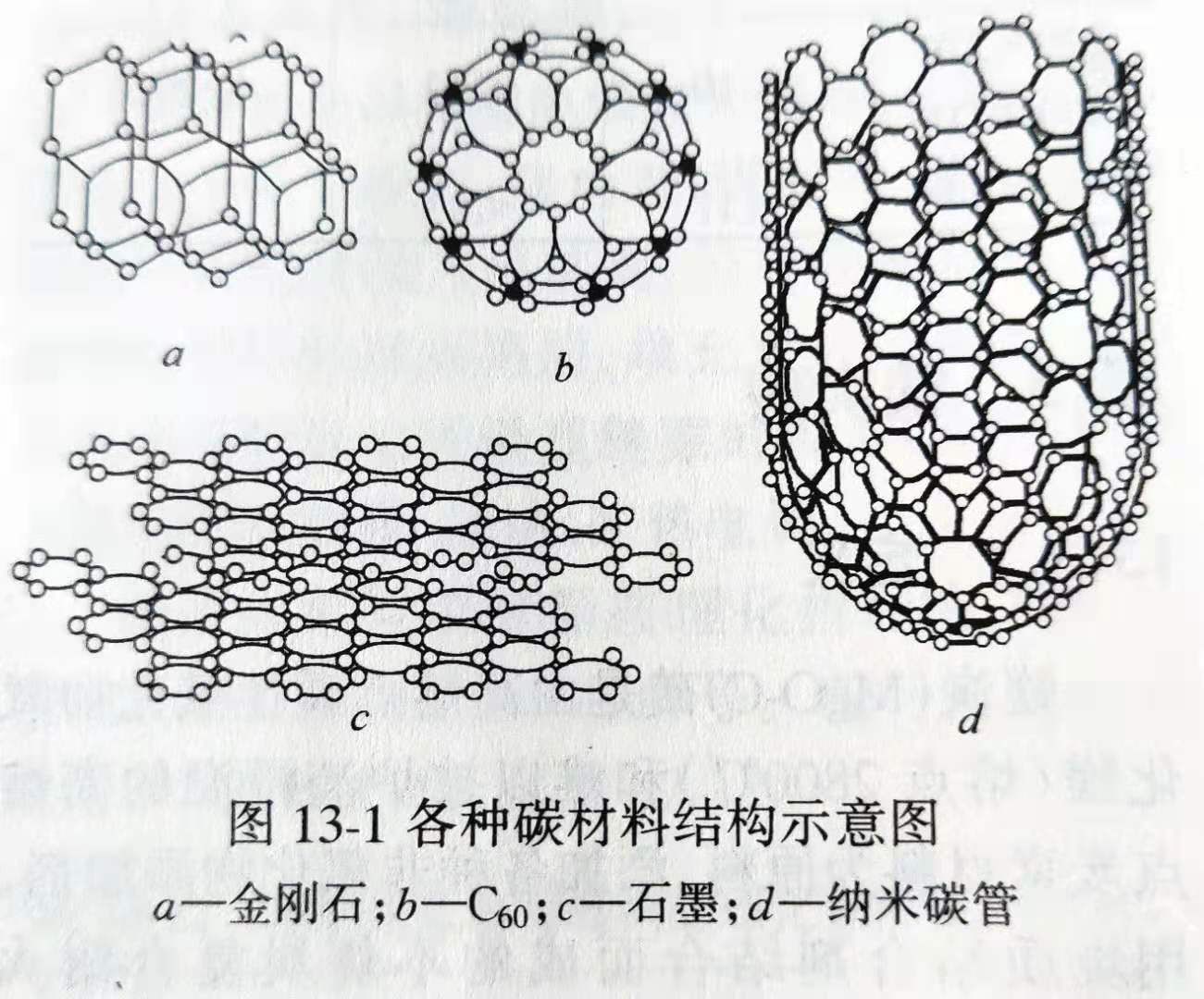
Crystalline graphite carbon is widely used in refractories. in addition, an appropriate amount of amorphous carbon (such as carbon black) will also be added to some products (such as aluminum-carbon products) to improve the composition of carbon-containing refractories and improve their properties.
Properties of Magnesia-carbon Brick
As a kind of composite refractory, MgO-C brick effectively makes use of the strong slag erosion resistance of magnesia and the high thermal conductivity and low expansion of carbon, and compensates for the biggest disadvantage of poor spalling resistance of magnesite.
resistance to elevated temperatures
TM.PMgO=2825℃,TM.P grapgite﹥3000℃There is no eutectic relationship between MgO and C at high temperature. Therefore, magnesia-carbon refractories have good high temperature properties.
Strong slag resistance
MgO itself has strong corrosion resistance to alkaline slag and high iron slag. The wetting angle of graphite to slag is large and the wettability of slag is poor. Therefore, magnesia-carbon refractories have high slag resistance.
Good thermal shock resistance
Thermal shock resistance index of materials
R∝Pmλ/Eα
Pm-The mechanical strength of material;
λ-thermal conductivity of materials;
E-The elastic modulus of a material.
α-The coefficient of linear expansion of the carbon-material.
Graphite has a high thermal conductivity (lambda 1000℃ graphite =229W/m·K, lambda 1000℃MgO=24.08W/m·K), a low coefficient of linear expansion (alpha 0~1000℃ graphite =1.4~1.5×10-6/℃, alpha MgO=14~15×10-6/℃), a small modulus of elasticity: E=8.82×1010Pa, and the mechanical strength of graphite increases with temperature. Therefore, magnesium-carbon brick has good thermal shock resistance.
High temperature creep is low
Compared with other ceramic - bonded refractories, mgo-c brick shows excellent creep behavior. This is because the matrix of mgo-c brick is composed of high melting point graphite and magnesia fine powder, and there is a solid carbon binding network between the particles, which is not easy to produce slippage. There is no eutectic relationship between C and MgO, and there is little liquid phase.
Effect of raw materials on properties of magnesia carbon brick
The main raw materials for mgo-c brick production are magnesia, graphite, binder and additive. The quality of these raw materials directly affects the performance and use effect of mgo-c brick.
magnesia
Magnesia is the main raw material for mgo-c brick production. The quality of magnesia has a very important impact on the performance of mgo-c brick. How to choose magnesia reasonably is the key to the production of mgo-c brick. Magnesia is composed of electrofused magnesia and sintered magnesia, which have different characteristics.
Electrofused magnesia: > 80 microns, less impurity, less silicate phase, higher direct bonding of grains, less grain boundary.
Sintered magnesia: grain size is small (0~60 microns).
In addition to chemical composition, magnesia raw materials also require high density and large crystallization in terms of organizational structure. Therefore, as the quality index of magnesia raw materials for mgo-c brick production, the following contents should be investigated:
(1) MgO content (purity);
(2) types of impurities, especially C/S and B2O3 contents;
(3) density, pore diameter and pore shape of magnesia (sinter).
The purity of magnesia has great influence on the slag resistance of mgo-c brick. The higher the content of MgO, the less impurities, the lower the degree of silicate phase segmentation, the higher the degree of direct bonding of calcite, the better the slag penetration resistance and slag melting ability.
The impurities in magnesia mainly include CaO, SiO2, Fe2O3, B2O3, etc. The content of B2O3 impurities in natural magnesia is very low and belongs to borax free. If the content of impurities in magnesia is high, especially the B2O3 compound, it will have an adverse effect on the refractability and high-temperature performance of magnesia.
The impurities in magnesia mainly have the following adverse effects:
(1) reduce the direct binding degree of calcite;
(2) formed low melt with MgO at high temperature;
(3) at 1500~1800℃, impurities such as Fe2O3 and SiO2 react with C before MgO, leaving pores that make the product's slag resistance worse.
(4) for magnesia raw materials, in addition to the total amount of impurities, the type and relative content of impurities also have a significant impact on the performance of magnesia. Among them, CaO/SiO2 ratio and B2O3 content have the most obvious influence. CaO/SiO2 ratio controls the sub-crystalline phase type of magnesia. Generally speaking, magnesia refractory requires CaO/SiO2 ≥ 2, so that the phase composition of the sub-crystalline phase falls in the high melting point zone between CaO- mgo-c2s in the tri-phase diagram of CaO- mgo-sio2, so as to improve the high-temperature stability of mgo-c brick, as shown in table 13-2.
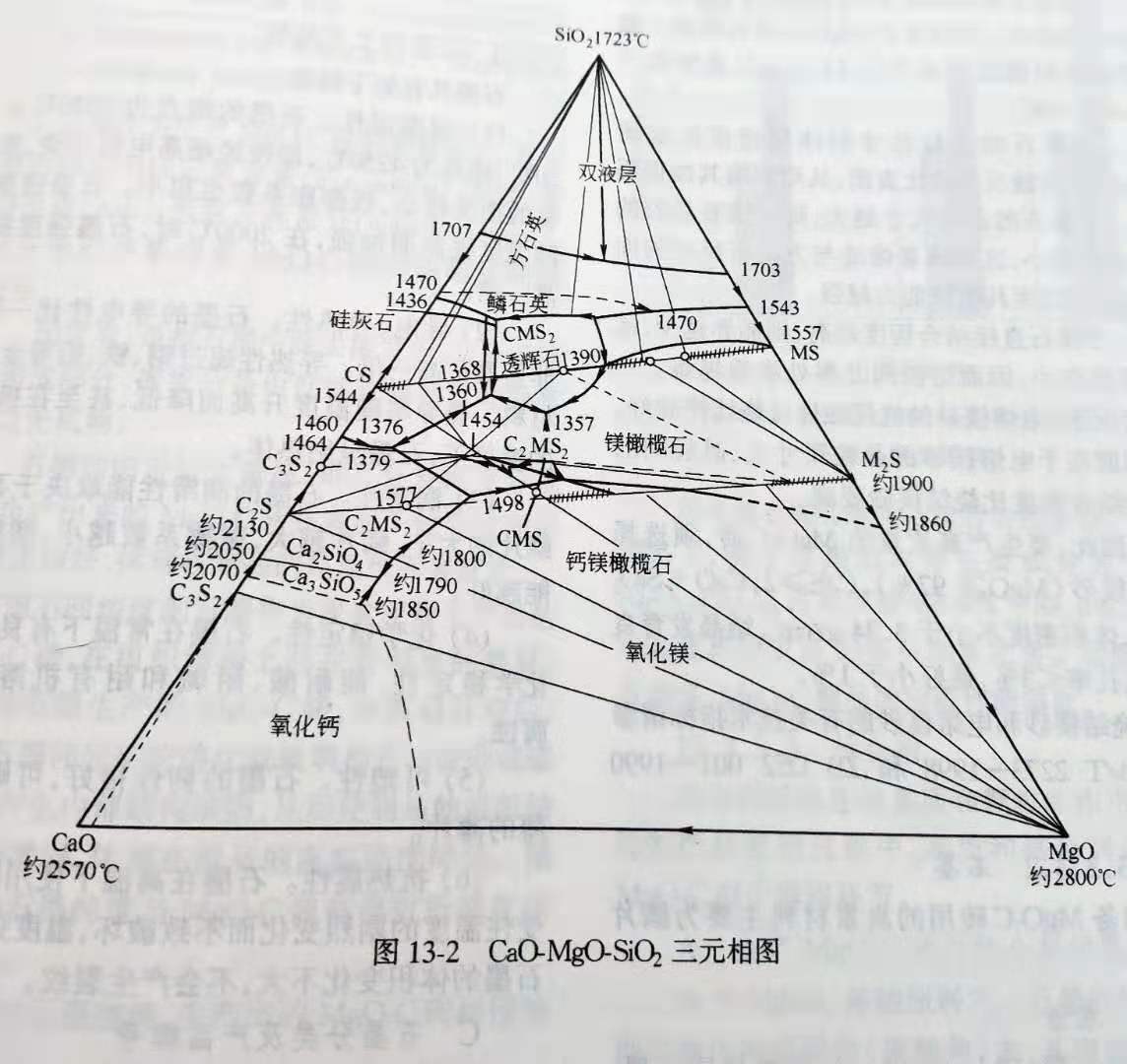
In the CaO-MgO-C2S three-phase region, the melting point of C2S (2130℃) and C3S (1900℃) is very high. In addition, low-silica magnesia can reduce the high-temperature reaction between MgO and C. Magnesia with high CaO/SiO2 ratio has a good stability of coexistence with graphite at high temperature. The higher the CaO/SiO2 ratio is, the higher the direct binding degree of calcite is.
The sintering property of magnesia also affects the properties of MgO-C brick. The higher the volume density of magnesia is, the less the closed pores are, the lower the solubility of magnesia into slag is, and the better the slag melting resistance of mgo-c brick is.
In the process of using mgo-c brick, one of the important melting processes of magnesia is slag erosion into the crystal boundary of magnesite, which promotes the reaction between MgO and slag. When the slag reacts with the impurities such as SiO2 and CaO in the grain boundary of calcite, the calcite crystal is continuously flayed into the slag. The high volume density magnesia can reduce the intrusion of slag and improve the corrosion resistance of MgO-C brick. Therefore, the production of mgo-c bricks generally requires the volume density of magnesia not less than 3.34g/cm3, preferably more than 3.45g/cm3.
The grain size and volume density of magnesite determine the specific surface of MgO reaction with slag, thus affecting the degree of corrosion. The larger the grain size of calcite, the smaller the grain size of calcite is relative to the surface, which means that the reaction area between slag and calcite is smaller, so its slag resistance is stronger.
The higher the degree of direct combination of magnesite, the less the grain boundary, the smaller the grain boundary area, thus the more difficult the slag penetration to the grain boundary. In general, the corrosion resistance of electrofused magnesia is better than that of sintered magnesia. The reason is that the grain size of electrofused magnesia is larger and the direct bonding between grains is higher than that of sintered magnesia. Therefore, in order to produce high-quality mgo-c bricks, it is necessary to choose high purity magnesia (MgO ≥ 97%),C/S ≥ 2, low amount of CaO+SiO2, no less than 3.34g/cm3, well developed crystallization, stomatal rate ≤ 3%, preferably less than 1%.
For technical specifications of sintered and electrofused magnesia, please refer to GB/T 2273-1998 and ZB D52 001-1990.

